SODIUM ADSORPTION RATIO
Introduction:
Previous pages have given various descriptions of sodium in
wastewater (and greywater) where the effects have been related to concentration
of sodium in the wastewater (milligrams per litre) or the load per wash
(grams per wash). While those are simplistic views of sodium and an easy way to
calculate the potential effect of sodium on soils and plants, there is one
measure of sodium that is related to other components of the wastewater.
Sodium adsorption ratio (SAR) is a ratio of the sodium (detrimental element) to the combination of calcium and magnesium (beneficial elements) in relation to known effects on soil dispersibility. Refer back to the photographs on the Garden page (click here) that show the soil response of slaking and dispersion. It is accepted that the SAR and the electrical conductivity of irrigation water can be assessed for the potential to cause dispersion in a soil. Sandy soils are not affected by the sodium (because of the low clay content), but the plants growing on them may be affected. So the following discussion relates mostly to loams and clays.
SAR is a mathematical relationship, set out in Equation 1, the concentration of sodium in relation to calcium and magnesium. The sodium comes from the raw water input, the clothes being washed and the laundry detergent. Because the concentrations of sodium, calcium and magnesium vary across capital cities throughout Australia, there are several graphs for these major water supplies, and for rainwater as well. Data for the metropolitan supplies have been sourced from each water agencies' web sites (see references below).
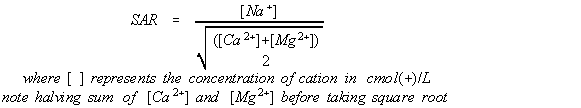
Equation 1
My PhD research (Patterson, 1994) showed that loss of soil
permeability commenced as low as SAR 3 when the electrical conductivity was
about the same from domestic wastewater. Internationally, SAR 6
is accepted as a level above with soil permeability and structural stability may
be affected. However, effects of high SAR are more dramatic at low EC and
one method of overcoming the detrimental effects of high SAR is to increase EC,
by either adding gypsum (no increase in pH) or lime (increases pH). The
level at which the increasing EC nullifies the SAR is called the threshold
electrolyte concentration after research by Quirk and Schofield (1955).
While a mathematical calculation is used to derive the threshold electrolyte
concentration, it can often be replaced by simple laboratory experiments.
Discussion of these calculations is beyond the scope of these pages.
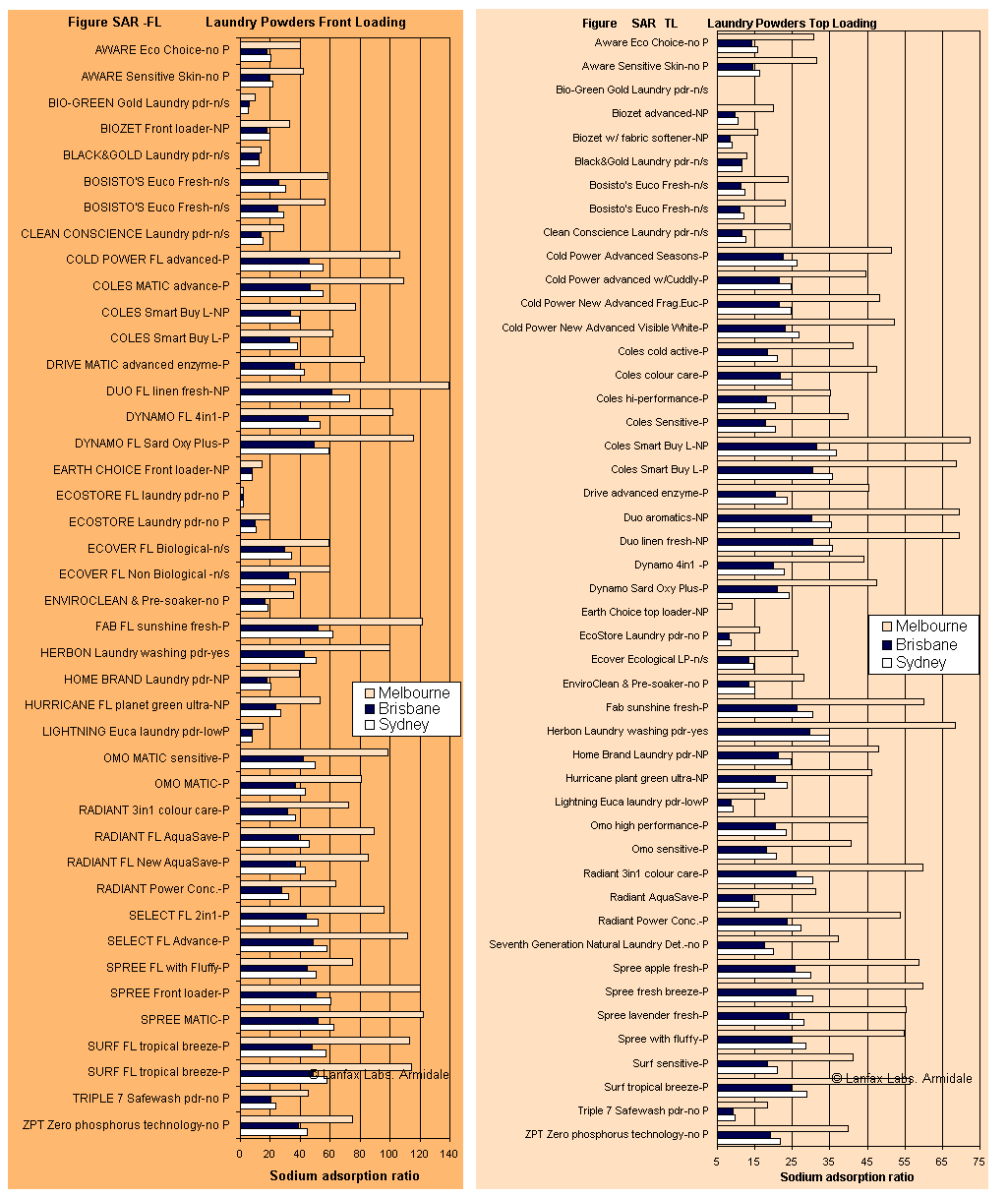
The graphs below were created from data obtained during the research of the laundry detergents using measured values of sodium, calcium and magnesium, together with the documented levels of each of these elements in the water supplies of Sydney (Prospect), Brisbane (Mt Crosby), and for Melbourne (City West Water).
The SAR of a detergent in rainwater is higher than in Melbourne's water because very few calcium and magnesium ions are present in rainwater, even though Melbourne's supply is very soft water, there is almost no calcium or magnesium to counter the sodium concentration. The front loader averaged SAR 117 and the top loader SAR 56 in rainwater.
So why are the bars longer if Melbourne has the softest water, and shortest when Brisbane has the hardest water? Because sodium adsorption ratio is a ratio of sodium to calcium and magnesium (see Equation 1), lower levels of calcium and magnesium in the raw water (therefore softer water) calculates to a larger number as the SAR. The likely effect is that the higher SAR may be more damaging to the clayey soil. SAR values over 15 are extremely hazardous when the EC of the wastewater is low, as is the case for most of these detergents. Fortunately some of the detergents have very high EC and this will assist in reducing the dispersibility of the soil subjected to greywater irrigation.
It is obvious from Figures SAR-FL and SAR-TL that there are products that have SAR < 15 in the metropolitan supplies, while other products have very much higher SAR in Melbourme water because of the softness (low calcium and magnesium) of that water. Note the higher levels of SAR in the front loader compared to the top loader - refer to the scale of the x-axis (bottom of graph).
In rainwater, most of the SAR values will be higher than those for Melbourne because there is almost no hardness in rainwater. When considering hardness, the harder the water, the more detergent that is required to overcome the negative effects of calcium and magnesium on the wash performance as discussed under the hardness page (refer back to that page). The role of the 'builder' in a detergent is to overcome the effects of hardness, so it stands to reason that the softer the water, the lower the dose required to produce an ideal wash. That important point is missed in the advertising on the product's packaging. Have you ever seen the recommendation to use less detergent in rainwater? I bet not!
The above graphs will be reproduced for the liquids when the data are available.
References:
Brisbane Water Quality: Mt Crosby and Enoggera results available. North Pine
(closed for this period)
http://www.brisbane.qld.gov.au/bccwr/lib169/aug2007_chemical_analysis_water_quality_results.pdf
Sydney Water Quality: North Richmond and Prospect
supplies
http://www.sydneywater.com.au/Publications/Reports/TypicalWaterAnalysis.pdf
Melbourne: City West Water -
http://www.citywestwater.com.au/about/docs/Water_Quality_Report_2007.pdf
PATTERSON, R.A., (1994) On-site treatment and disposal of septic tank effluent Ph.D. thesis, University of New England (see reference under Publications)
Quirk, J.P. and Schofield (1955). The effect of electrolyte concentration on soil permeability. J. Soil Sci., 6, 163-173