TOTAL ALKALINITY
Introduction
While Lanfax Labs have measured the pH of the detergents in
a wash water for a front loader (25 L) and a top loader (60 L), the pH
has been strongly alkaline in all cases (more than 9.0). meaning an excess of
hydroxide (OH- and HCO3-) than H+ ions
(acid). When the ions
in excess are hydroxide (OH-), the liquid is alkaline (maybe
called basic). Water is a weak electrolyte in which hydrogen ions and
hydroxide ions are in roughly equal proportions at pH 7. Rainwater may
have a slightly acid pH (pH 6), while reticulated water is usually around pH 7.8
(to protect the pipes and other infrastructure in the delivery system)>
When we talk about "TOTAL ALKALINITY" we are not referring to whether the liquid is acid or alkaline, but measuring the buffering capacity of a liquid that is already above pH 4.5. Buffering capacity is a measure of the ability of the liquid to resist change of pH. A liquid with high total alkalinity will take a lot of acid to reduce the pH below neutral (the acid zone), whereas a weakly buffered liquid will change to lower pH with only a small amount of acid. Total alkalinity is reported in units of milligrams per litre calcium carbonate equivalent (mg/L as CaCO3). The technical details are not needed at this point.
The best example of a well buffered solution is our blood. If after eating a meal or drinking alcohol the increase of acids or alcohols in the blood would change quite dramatically if the blood was poorly buffered. Fortunately, our blood is strongly buffered and it takes large amounts of alcohol, food or drugs to change the pH outside the range that is good for us. That's important to maintain our normal function.
In the environment, the buffering capacity of water comes about because of the varying amounts of hydroxide, carbonate and bicarbonate (from carbon dioxide dissolved in the water) and hydrogen ions. Strongly buffered liquids are difficult to shift pH. Water in the natural environment has a mild buffering capacity and may be easily disturbed by the entry of small amounts of acid or alkaline substances.
Laundry detergents are, in the main, alkaline, that is their pH in water is above 7, maybe as high as 11 (strongly alkaline). We can also call them basic liquids which derive their buffering capacity from hydroxide, carbonate and bicarbonate. Sodium carbonate (washing soda), sodium bi-carbonate (baking soda) and sodium hydroxide (caustic soda) are commonly used in detergents to soften the water and increase the pH (grease, fats and food dissolve better in highly alkaline liquids). Phosphates, borates and silicates present in laundry detergents can also affect total alkalinity.
Rainwater has a very low buffering capacity (total alkalinity less than 10 mg/L CaCO3), groundwater from bores in limestone areas or under basalt landscapes may have high total alkalinity (above 500 mg/L CaCO3) from the carbonates dissolved in the water.
Good quality garden soil has a strong buffering capacity but strongly alkaline laundry water may disrupt that equilibrium.
In our reticulated water supply, the total alkalinity of the treated water is increased to about 80-100 mg/L CaCO3 to prevent the pipes dissolving in the water. That would happen if we were to be supplied with very clean water (very low buffering capacity).
When a strongly buffered laundry water (high total alkalinity) is discharged to the environment, two outcomes may result:
1. The pH of the laundry wastewater (greywater) is affected by the pH of the environment to which it is released and ideally should have little effect on changing the pH of that environment. It is the laundry detergent's pH that is neutralised, rather than the soil becoming more alkaline. This happens with poorly buffered detergents, they have a low alkalinity. Liquid detergents mostly fall into this category.
2. The pH of the laundry wastewater (greywater) remains unaffected by the receiving environment and actually changes the receiving environment to become more alkaline - this may not be a good outcome. Liquids with high alkalinity are more likely to change the environment and may seriously affect plant growth. This may occur in soils where the high alkalinity of the detergent makes the soil alkaline. Powder detergent are more likely to have this outcome.
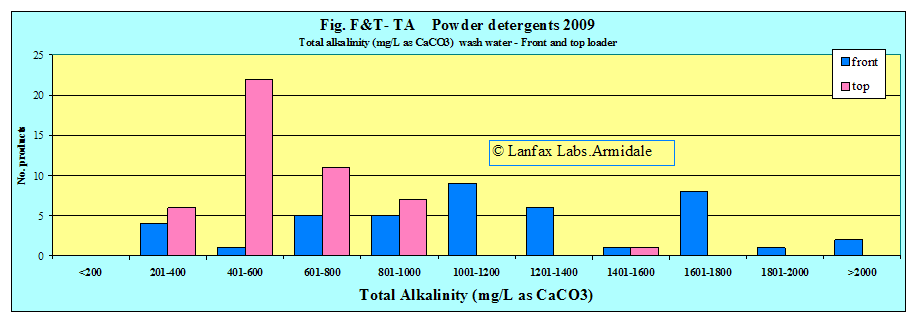
In the analysis of the liquid and powder detergents, the powders have higher total alkalinity than the liquids.
The following graphs show the alkalinity of laundry powders, mixed at the two rates.
INTERPRETATION: The longer the bar, the higher the total alkalinity and greater the influence the laundry wastewater will have on the natural total alkalinity of the soil and its reaction against change to the natural buffering capacity. The longer the bar, the greater the chance that the wastewater will have a detrimental effect upon the pH of the soil, particularly with repeated applications.

ęCopyright: Lanfax Laboratories PO Box
4690 Armidale NSW 2350.
Contact: Dr Robert Patterson
+61 2 67751157 email:
lanfaxlabs@bigpond.com.au