pH measurement
pH is a measure of the hydrogen ion activity (electron donor). pH 7 is neutral, values lower are acid (high H+), while values above 7 are alkaline. As the values are logarithmic values, pH 7 has 10 times the activity of pH 6, and pH 10 has 1000 times the activity of pH7. There are no units of measure for pH, it is a numerical value.
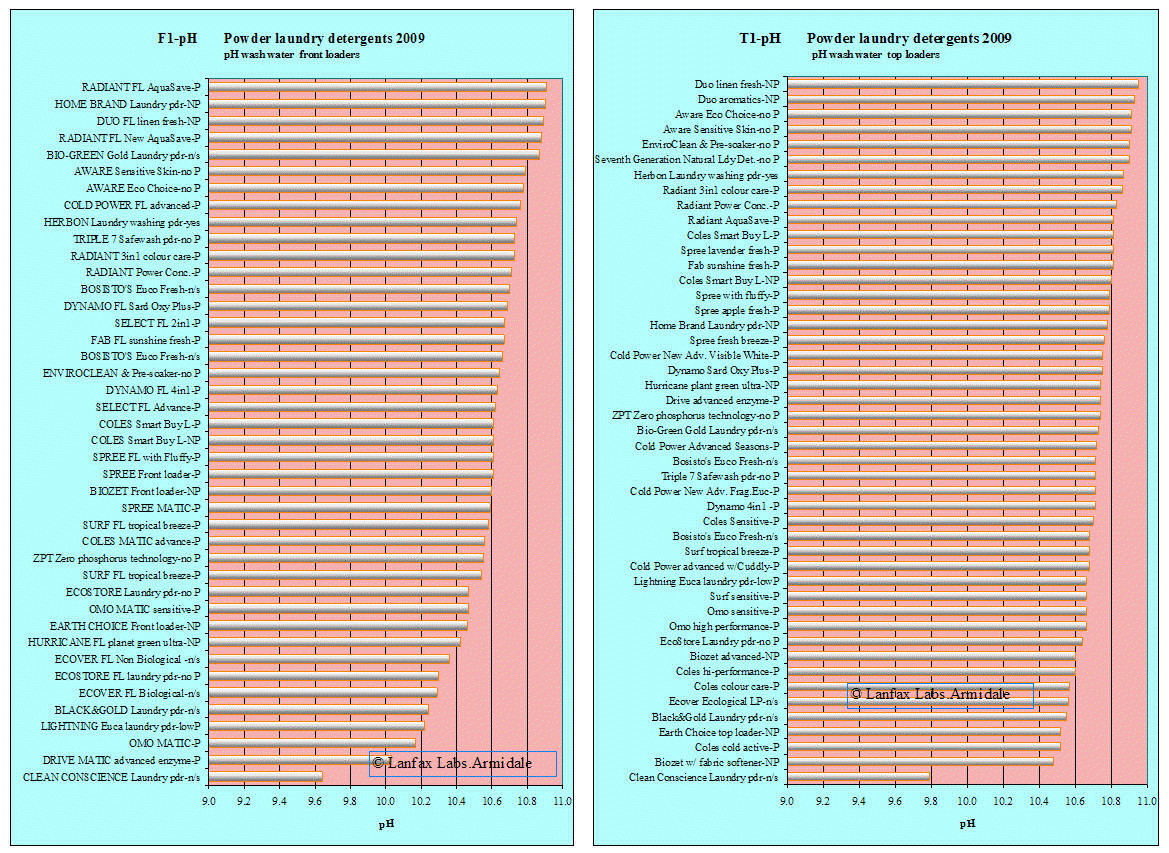
The pH of the wash cycle water, that is 25 L for the front loaders and 60 L for the top loaders was measured, using a freshly calibrate pH probe and meter, on the sample within one hour of mixing the detergent with the water at the dose for a normal wash as recommended by the manufacturer. The pH of your wash water may vary where you are using a town water supply that has a higher starting pH than rainwater (used in this research).
Detergent was mixed at one rate for the front loader and the other recommended rate for the top loader. Some detergents were recommended only for front loaders ( 24 products labelled FL and MATIC in block letters), while 18 others were recommended for both (front and top) or for top only. Where the products were recommended for both, the results will be shown in both graphs, each at the representative rate for the machine type.
The measurement of pH is important when discharging washing machine water either to the land or to a sewer. High pH (alkaline) has the ability to dissolve organic matter (sweat, blood, food) and that includes skin. One should be careful when handling the wash water when pH is above 10. The longer the bar, the higher the pH (more alkaline). High pH may cause some plant nutrients to become less available, others more available, and leads to the dissolution of organic material. High pH may also induce dispersion in the soil (since that is their role in the detergent) to which the washing water is discharged. pH levels of wastewater outside the range of 5-9 may be detrimental to the environment.
High pH liquids act as dispersing agents, causing the soil particles to separate and may lead to soil structural decline. The normal pH range for biological activity is between 5 and 9. All these products are highly alkaline and well outside the range at which normal biological activity is expected to perform. They are "hazardous" products and cannot be labelled "environmentally friendly", quite the opposite.
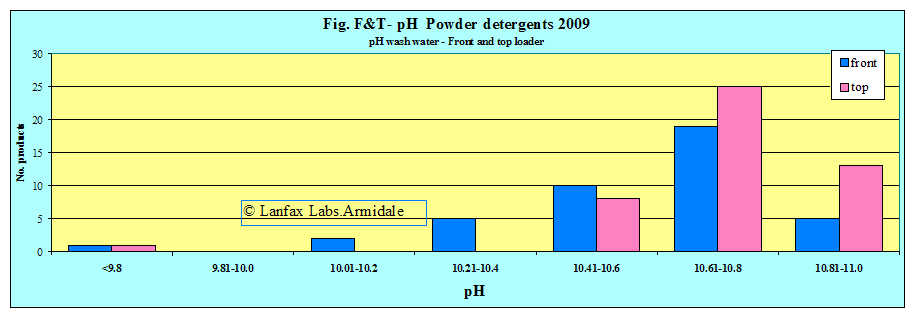
Notice in Figure F&T-pH that the majority of the products are clustered around the 10.6 to 10.8. A few powders mixed at the front loader rate are less alkaline.
Summary
| Source | Lowest pH value | Average pH value | Highest pH value |
| pH front loader wash (42 samples) | 9.64 | 10.57 | 10.91 |
| pH front loader specific (24 samples) | 10.03 | 10.55 | 10.91 |
| pH top loader wash (47 samples) | 9.79 | 10.95 | 10.71 |
So what's it mean?
Whether powders are used for front loaders or top loaders, the pH
of the wash water is generally very alkaline, that is has a pH above 8.5. Most of the powders represented in the two figures above have pH
values that are cause for concern because they fall well outside the range of
normal biological activity, and in all cases higher than desirable. While
the high pH is beneficial for removal of organic matter from clothes (food
stains, blood, sweat), it may also lead to the premature aging of clothes, that
is, they wear out because of the high pH rather than because of the washing
action.
As it is not possible to carry out normal clothes washing activities without some contact of the washing water with one's hands (be careful about splashing the wash water in your face), the potential for burns from the highly alkaline water may be high. Take care! See the instructions on the packet. Certainly keep these products away from children and animals.
When highly alkaline greywater is discharged to a land application area, the high pH may interact with the soil particles and dissolve organic material that can then be leached out of the soil. This is easy to demonstrate with any highly alkaline solution. The organic materials that are easily dissolved are the compounds that will, in time, degrade to become plant nutrients, so a loss of organic material may be detrimental to plant health.
Since some plants prefer acid environments, the highly alkaline washing water may be more detrimental than any of the other compounds in the washing water. If you are using greywater on your favourite garden, it may be prudent to test a small section with the laundry water before watering the whole garden with the greywater.
Unless the clothes are well rinsed, the carry-over of some of the chemicals may lead to the highly alkaline salts affecting human skin with which they make contact. Whether the standard test for rinsing is satisfactory on some of the less soluble products or those high in dissolved salts has yet to be documented.
ęCopyright: Lanfax Laboratories PO Box
4690 Armidale NSW 2350.
Contact: Dr Robert Patterson
+61 2 67751157 email:
lanfaxlabs@bigpond.com.au