Reuse of Greywater on
Gardens
If there is other information you would
like to see here, please contact Lanfax Labs
Introduction: The reuse of greywater on gardens can be a valuable resource in times of drought but can also be hazardous to plants and soils. The page on GREYWATER suggested some of the properties that can be attributed to various laundry detergents. Other properties come from the quality of the drinking water into the home before it is contaminated as greywater. Not all town water supplies are the same. There is no standard for drinking water but there are Australian Drinking Water Guidelines (2004) that provide a range of microbiological, chemical and physical properties that are expected for drinking water. Unfortunately it is a fact of location that water supplies along the eastern seaboard (the narrow strip between the ocean and the Great Dividing Range) have water with significantly lower dissolved minerals than town water supplies to the west of the range. This difference will be discussed when we talk about salinity. If on the other hand you are using rainwater, there is only minor salinity in the water and nearly all the contamination will come from the chemicals used in the home.
The most important considerations for reuse are:
the quality of the greywater;
the plants you will irrigate with the greywater;
the soil in your garden;
how much greywater you spread over a given area (measured in litres per square metre);
how often you irrigate the same area; and
when it rains on the garden; and (I'll explain how the most damaging effect of greywater reuse can be from rain, but more of that later.)
amelioration of the irrigation area.
So let's look at the reuse of water on the garden in some logical manner and follow the points outlined above. Of course you will need to consider your individual aim of reusing greywater, whether it is just to keep plants alive until the drought breaks, or as a permanent means of reducing your reliance on drinking water (town water or tank water).
Remember - there is a difference between SALINITY (all salts) and SODICITY (sodium specifically). Many salts are good for the garden and we will discuss the use of some of these in ameliorating the soil. Sodium usually leads to loss of soil structure and plant vigour (even death).
QUALITY OF GREYWATER - to be
completed
The drinking water that comes into your home may be from town
water supplies (treated, reticulated water that may be from rivers, bores or a
mixture then treated with chemicals to remove suspended material, change the pH,
amend the alkalinity and reduce the hardness as well as adding chlorine and
fluorine); rainwater collected on roof tops and stored in plastic, galvanised or
concrete tanks; or bore water from basalt, sedimentary or basalt aquifers
(various proportions of sodium, calcium and magnesium). Depending upon the
source of water, you may already have an abundance of minerals to consider on
top of the chemicals you will add during its use within the home.
Town water supplies (reticulated water from
water treatment plant)
The quality of water supplied by local authorities through the reticulated
 networks varies from locality to locality and much is dependent upon the
landscape from which the water is collected. Some catchments have
very hard water (difficult to lather) that must be softened before reticulation
(water high is calcium and magnesium has those elements precipitated out), while
other catchments may have high sodium that cannot be removed by precipitation
and filtration.
networks varies from locality to locality and much is dependent upon the
landscape from which the water is collected. Some catchments have
very hard water (difficult to lather) that must be softened before reticulation
(water high is calcium and magnesium has those elements precipitated out), while
other catchments may have high sodium that cannot be removed by precipitation
and filtration.
Other chemicals are added to the water during treatment to assist in flocculating clays (aluminium salts) so that they can be removed by filtration, while other chemicals are added to adjust pH, to add fluoride and chlorine. All these salts affect the final product that you receive as reticulated water and have been developed with consideration of health and aesthetics and nothing at all to do with any potential future use of the water. In the same way that most household chemical manufacturers don't consider how the water contaminated with their products may be used to irrigate soils.
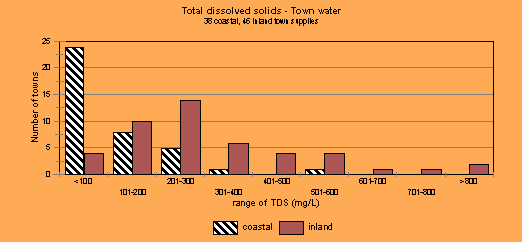
From the figure above it is clear that the coastal town water supplies in NSW mostly have low levels of total dissolved solids (TDS), which can also be referred to as salinity (not sodicity) and the inland towns, those over the Great Dividing Range to the west, mostly have higher TDS. So all water cannot be treated the same when it comes to judging what happens to the water when you turn it into greywater in the home. The harder the water, the more detergent you have to use to overcome the effects of the salts causing hardness before you even start to wash the clothes.
Rainwater generally has TDS less than 20 mg/L and to use the same quantities of laundry detergent as reticulated supplies is simply a huge waste of detergent. Unfortunately, I think the laundry product manufacturers base the quantity of detergents recommended for the urban areas and are not dealing with rainwater quality.
Bore water or well water will be strongly influenced by the geology of the aquifer (water bearing stratum) from which it was sourced. Bore waters are generally higher in TDS than town water supplies (except for some very special aquifers). Some bore water would be better not used in the laundry because of the increased use of detergents that have to be use and the potential that greywater will have for future plant and soil problems.
PLANTS IN YOUR GARDEN
It is not the intention of this website to
give suggestions of plants that are suitable for greywater irrigation because
climate, soil types, and garden care will play more significant roles and these
vary so widely across the country. What you need to consider is whether your
garden has (a) been developed using low salinity town water and you only want to
use greywater as an interim resource until water restrictions are lifted, or
whether (b) you want your garden to be sustainable on greywater even when water
restrictions are lifted. There is an economic incentive to reuse
greywater, but it must be used wisely or it will be a hazard to your garden.
Many plants are intolerant of high salinity and even more plants are sensitive to sodium and that is because sodium affects the osmotic pressure in the plant cells (the ability to move water and minerals from the soil into the plants cells and hold the cells rigid, rather than move nutrients from the plant cells into the soil solution). Plants that are irrigated with water high in sodium will look like they need a drink, and in severe cases the leaf margins (around the edges of the leaf) will start to look scorched or burnt. When that happens you have serious problems and using rainwater may not be the option as rainwater can make the problem of sodicity very much worse.
The amount of phosphorus in the greywater may not be a problem as only a few plants are sensitive to high phosphorus levels. In many soils there is a natural mechanism to immobilise the phosphorus and minimise the phosphorus available to plants from the soil solution. There is very little evidence that Australian native plants are intolerant to phosphorus, except for perhaps Proteaceae family, but that is a contentious issue for some gardeners. It is true though, that many Australian species have developed mechanisms to absorb phosphorus from soils that have exceedingly low natural levels of phosphorus, but when phosphorus is available, the plants thrive. If phosphorus kills native plants, then why is phosphorus as important to native plants as it is to all living things - including us?
When you discuss your plant selection with your local nursery, be specific when discussing sodium issues as this element will dominate in greywater. And don't let your nursery person tell you that sodium is the same thing as salinity - that is simply not true.
SOIL IN THE GARDEN
It would be unreasonable to think that only the quality
of the greywater and the plants were important when considering reuse of
greywater. More important is the soil type, soil horizons (the different
layers of soil as you dig deeper), soil
chemistry and soil microbiology. Let's not turn this into a soil science lecture, but there are
some topics you need to understand so that you can make informed choices as to
whether you reuse greywater or not.
Soil is graded in a couple of ways. First, by the proportions of sand, silt and clay that are the mineral components based upon grain size and that classification is called "Soil Texture" and secondly by the "Structure" - the way the soil particles are aggregated together.
SOIL TEXTURE - Simply we can refer to soils as sands, loams and clays and then give them descriptions like loamy sand, sandy loam, clay loam or light clay. So how do you tell what it is - that's simple. Take some soil in your hand, as much as you can hold, add water slowly while manipulating the soil in your hand until you can work the soil into a ball, about the size of a golf ball.
SANDS - difficult to
form into a ball, you can see sand grains.
LOAMS - easy to form into a ball, and the ball is rather
smooth and spongy, not very sticky.
CLAYS - form into a ball that behaves like plasticine,
sticky and you can roll some out into very fine ribbons
It is just wishful thinking that soil classification is as simple as that - there's another couple of steps to determine the SOIL TEXTURE, but essentially we are after the texture (feel) and behaviour of the moist soil.
The second classification we need is the SOIL STRUCTURE, that is, how are the particles in the soil glued together to form larger lumps, blocks, clods into what we call aggregates (or peds).
Simply dig a hole about spade width and spade depth, put all the soil to one side. Then down one side of the hole, take a spade full of soil about 75 mm thick, lift out that wedge of soil, and from about waist high, drop the soil onto a hard surface. Sort the soil into several grades - Move all the larger lumps towards one end, and all the mid size lumps into the middle and all the smaller lumps to the other end. The figure opposite, from NZ field manual is an excellent illustration of how the soil can be classified.


Left hand photo: Wedge of soil dropped on ground (Photo: R.Patterson). Right hand set: Reference: Soil Management Guidelines for Sustainable Cropping. TG Sheppard, CW Ross, LR Basher and S Saggar. Landcare Crown Research Institute, NZ. 2000
SANDY soils will not behave in the same way as the photos above because they are not strongly aggregated as in soils that have a moderate to high clay content.
The difficulty with SANDY soils is not a chemical problem but one of water moving through the soil too rapidly with insufficient time for the nutrients to be absorbed by the plants or adsorbed onto the soil minerals. On sandy soils the area has to be increased to account for the poor soil nutrient status - you need to spread the greywater further so the plants can access the nutrients before they leach deeper into the soil profile.
SPREADING WATER OVER GARDEN
The area over which you spread the greywater depends upon the salinity, sodicity and phosphorus levels in the greywater. Forget about nitrogen because these levels are very low and significantly less than the plants require, even if spread by bucket over a small area. Plants require about 25 g N/m2 (read that as 25 grams of Nitrogen per square metre), 3 g P/m2, and about 40 g Na/m2. These levels are guides only and vary from soil to soil, although the levels are very similar for most plants, except for sodium (Na) for sensitive plants.
How do you calculate the area over which you should spread the greywater? As these calculation can be difficult without having a lab analysis of both the greywater and the soil, in the table below are some examples of what area you may need to irrigate if you choose various detergents with low, medium or high phosphorus. The same has been done for sodium levels. This is a guide only and is based upon the laundry water contributing the major portion of these two elements. It is assumed that the family will do seven washes per week.
Table G1. Suggestions for application areas for various levels of phosphorus or sodium based upon a clay soil.
| FRONT LOADER or TOP LOADER | |
| element in detergent | area to spread water over each year |
| low phosphorus (<1 g/wash) | 122 m2 |
| medium phosphorus (2-3 g/wash) | 240 - 370 m2 |
| high phosphorus (>5 g/wash) | 600 m2 |
| low sodium (<10 g/wash) | 90 m2 |
| medium sodium (20-30 g/wash) | 200 - 300 m2 |
| high sodium (>50 g/wash) | 500 m2 + 100 m2 each additional 10 g/wash |
As you can see from the table, you will always need much more land to spread the greywater because of the sodium compared with the phosphorus.
The above Table G1 is a "best guess" for permeability in soils that have a loam surface soil over a clay subsurface. The permeability of the loam is expected to be reasonable while the permeability of the clay would be very slow. Therefore the loading rate has to be less than the ability of the topsoil to infiltrate water.
As you can see from Table G1, attempting to bucket water over the garden so that the greywater is spread evenly over the required area will be a mammoth task and one that will become very tiring. Even to spread the greywater using an irrigation system will require considerable irrigation pipe and an appropriate pump.
There is a difference between "infiltration" and "permeability" and they do not mean the same thing. Infiltration is just a measure of how quickly water moves from the surface into the soil. Throw a bucket of water over the soil and see how quickly it disappears. Of course, it will infiltrate quicker if the soil is moist than if it is already wet. Some soils will be difficult to wet when they are very dry, and mulch often becomes water repellent and can be more harm than benefit to the plants. Permeability on the other hand is the rate that water moves through the soil and is a quality that we cannot see. We need special devices to measure this property.
For SANDY soils, the application area has to be larger than the areas given in Table G1 because of the rapid permeability and the potential for the salts to move rapidly downwards to groundwater. Now that seems strange because the water will infiltrate into sands at a faster rate and you would think the area could be smaller. Well, that's only part of the solution, because what you are looking at achieving is spreading the chemicals (nutrients) in the greywater so they do not damage the groundwater or the plants.
FREQUENCY OF IRRIGATION
How frequently you go back to the same area depends again on the strength of
the greywater and the soil type. Table G1 infers that the water is spread
evenly over the whole area in a year. When the greywater is very strong (high salinity, high
sodium, or high phosphorus) the frequency has to be sufficient to ensure that
the salts are leached through the soil by using excess greywater or waiting for
rain. The
problem is that in a home garden you cannot know when the salts are leaching out
of the root zone. Therefore, more water is better. Except, the problem then
arises when rainwater falls on the soil and is likely to mobilise the sodium and
lead to soil structural problems. Now we are caught in a situation where
we may be causing problems by irrigating too much, or problems from not
irrigating enough. Where do we go?
Simple. If you are using a high sodium detergent, spread the greywater as far as you can - perhaps even further than in Table G1. You may need to ameliorate the soil with gypsum, lime or organic matter (composted materials) to reduce the loss of soil structure, as discussed below.
RAIN ON THE GARDEN
The real problem with using greywater that is high in sodium is that soil
structural problems that arise are really difficult to fix. The first sign of
structural problems is that the surface of the soil sets a hard crust - harder than
areas not irrigated with greywater. Another sign is that the greywater takes longer and longer to soak into the soil or that
rainwater is now ponding in areas it has not ponded before. When digging
in gardens that have been poorly treated with greywater, the soil will be very
cloddy (that is, more cloddy that soil that has not received greywater). You should always be seeking simple answers - how has the soil changed
after using greywater?
Soils high in sodium are likely to disperse in rainwater (middle photo below). The reason is complex and beyond the scope of this page to explain in scientific terms. What happens is that the individual clay particles separate and move into the soil solution and stay in suspension in the same way that smoke stays in the atmosphere for a long time. The individual clay particles can then move with moving soil water and these particles can block soil pores in the same way that a filter is blocked with solids. Finally, the soil will become so blocked with clay particles from above that the soil as a filter is blocked and water can only pass through very slowly, sometimes not at all and the soil becomes impervious. Dispersible soils have very hard-setting surfaces and this may be more pronounced when drying after rain rather than after greywater application.
You can test whether your soil is likely to disperse when rain wets the soil. Take some small soil aggregates, about 3-5 mm in diameter and dry them on some paper towel on the kitchen window sill. When they are dry, place them in a tumbler of clean water.
Emersons
Aggregate Stability test
Unstable aggregates (left hand photo) usually slake (simply fall apart) because they have insufficient organic glues to aggregate the soil particles and slaking is typical of subsurface soils that are low in organic matter. This is not a serious problem provided there is sufficient organic matter for the particles to re-aggregate on drying. Soils that disperse (middle photo) are real problem soils and most of these soils have problems because of high sodium. The water stable aggregates (right hand photo) either do not change their size or shape, or may swell but maintain their shape. Both these soils (class 7 and 8) are the types of performance we desire in good garden soils and require good management and plenty of organic material to encourage earthworms and the numerous species of bacteria and fungi that live in a favourable environment. Adding greywater may cause Class 7 or 8 soils to become class 1 or 2 soils, a most undesirable outcome.
This is a simple test you can do for yourself. Take three to five air-dry ped, each about 3-5 mm, and place in clean water and repeat the test in some greywater from your laundry wash water. The response you get will determine whether you need to (a) do nothing to the soil; (b) ameliorate the soil to overcome the effects of the greywater; or (c) avoid using greywater.
While it is beyond the scope of these pages, the real problems with spreading greywater on gardens comes when it rains rather than when the greywater is being applied. Thus, while ever it remains dry and we continue to irrigate with greywater we may think there are no problems. Then when the rain comes and we stop using greywater - be aware! Look for signs of water ponding in areas where it has not ponded before and signs of water stress in the plants. You may need to ameliorate to return the soil to its former status.
AMELIORATION
OF IRRIGATION AREA
Like all good stories, this one is not the miserable outlook that has been
portrayed above. There are ways of remediating an area that has been ruined by
highly saline or highly sodic greywater. There's usually not too many ways that
the soil will become unusable because of high phosphorus from greywater
irrigation, but sodium is a different matter. Don't be mislead by those who
suggest that high phosphorus kills native trees. Other than Banksia
species and a few similar species, all natives will respond favourably to
additional phosphorus, just the same as lawn and exotic plants respond.
Phosphorus is an essential plant nutrient - low phosphorus means slow growth -
but salt - yuk!
The effect we saw from having high sodium (middle photo above) is really one that has high sodium relative to calcium and magnesium. Therefore to ameliorate the area, we can add calcium or magnesium salts in the form of agricultural lime (calcium carbonate), gypsum (calcium sulphate) or dolomite (calcium & magnesium carbonate). We do not use hydrated lime (brickie's lime) because it will dissolve organic material in the soil and elevate the pH to alkaline conditions.
How much lime or gypsum do we use? That's another answer that depends upon soil type and the effect that sodium will have on the soil. Sandy soils don't need any because the sodium can be easily leached through the sandy soil with excess greywater or rainfall. Clay soils depend upon the type of clay and the impact that sodium has on the dispersion, but as a guide anything less than about a cup per square metre is simply not enough. Somewhere between one and 3 cups full per square metre will do no harm. Some soils require about four cups per square metre. Simply try a small area at one cup per square metre and see the difference.
We use gypsum where we do not want to elevate the soil pH. Gypsum adds calcium and sulphur to the soil (both plant macro-nutrients) without changing the soil pH but gypsum does change soil salinity. In this case the soil salinity is a benefit because the calcium helps to dislodge the sodium so that it can be leached by rainwater. Lime, however, adds calcium but also increases pH towards being more alkaline, as well as increasing salinity slightly. How much it shifts the pH depends upon the application rate and the soil properties. You will need a lab test to determine the exact amount of lime required.
Dolomite is used in place of lime, or with lime, when you need to add magnesium. Dolomite behaves the same as lime, elevating the pH as well as increasing salinity.
WHAT'S THE DIFFERENCE BETWEEN
FERTILISERS AND GREYWATER
The difference may not be very much. The elements in fertilisers are, ideally, soluble in water and the elements in greywater are
already dissolved in the water. But the big difference is that not many
fertilisers have the same proportion of sodium that your greywater is likely to
have. Most fertilisers have very little sodium because sodium is so detrimental
to plants.
Many valuable garden fertilisers (as distinct from superphosphate used on farming lands) have various ratios of nitrogen, phosphorus and potassium (see ratios of N:P:K on the label) because these elements are required in reasonably large amounts and are called macro-nutrients. Sulphur, calcium and magnesium are also macro-nutrients. Other nutrients are required in minute doses and include zinc, iron, copper, boron, molybdenum. So we can buy fertilisers that have various mixtures of these macro and micro-nutrients, fertilisers to suit roses, vegetables, lawns, orchids and other specific groups of plants.
Unfortunately the manufacturers of laundry products are not so kind as to put other elements in the laundry products that your plants require. Indeed many laundry product manufacturers do the exact opposite. They use large amounts of sodium that are detrimental to soils and plants. There's almost no nitrogen or potassium in laundry detergents and calcium and magnesium would be detrimental to laundry detergents because they make the water "hard" and block out the effect of the detergents. Therefore, greywater has very little of the positive attributes of fertiliser other than the phosphorus and perhaps sulphur (in powders). So if you choose a detergent that is low in phosphorus, there's not much other than the water that is beneficial for the plants. And if you choose a laundry detergent that is low in phosphorus and high in sodium, then your garden will start to look pretty sick in a short time.
If you consider that a 50 kg bag of superphosphate (11% P) is worth about $40, then that is equivalent to 15 years worth of greywater from a detergent that has 1 g P/wash, so the phosphorus in the detergent is a mighty expensive way of putting phosphorus on your garden. But putting the greywater on the garden is better than discharging the greywater to sewer.
The other consideration is that when you use fertilisers, the N:P:K:S ratio is blended to suit particular plant groups and an ideal ratio for most plants is 100 N : 1P : 1 K and 1S with very low Na. In greywater you are likely to have very low N, a bit of P, almost no K and heaps of Na.......so greywater is not a fertiliser and never will be!
ADD FERTILISERS TO YOUR GREYWATER
IRRIGATION AREA
Now this may sound a stupid suggestion because many people think that the
components in greywater are plant nutrients. Well, as explained above, there are
very few essential plant nutrients in greywater and a lot of nasty sodium. Most of the benefit you see from using greywater is from the water, not what is
in it. Because the extra water is encouraging the plants to grow, then
plant nutrients must also be available if the plants are to grow well. You
will need to add nitrogen, some more phosphorus, potassium and calcium. A
good N:P:K mix from your nursery supplier, or some soluble fertiliser with a
small application of gypsum would be wise. On sandy soils, small
applications often and on clay soils one or two applications per year. Don't
think that you can get away without additional fertilisers - the plants won't
like you for that behaviour!
This advice is offered in general terms. Your specific requirements will have to be addressed according to the greywater application rate, the climate, soil type and plant species.