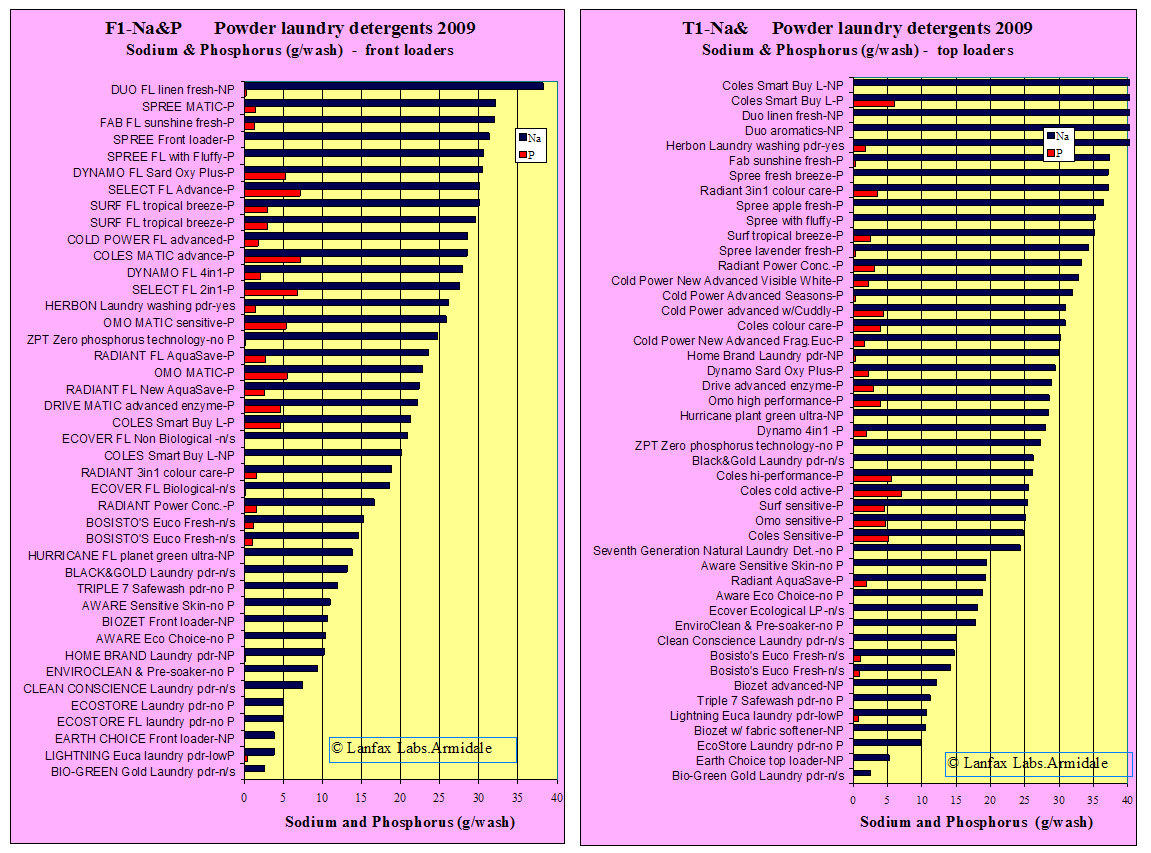
Sodium and Phosphorus
The data for sodium and phosphorus have been combined in the graphs below, one for front loaders (42 products) and the other for top loaders (47 products).
The purpose of combining the graphs is to allow choice of products depending upon your use of greywater. Where the greywater irrigation is to be on sandy soils, the impact of sodium is not as important as the loading of phosphorus. On sandy soils, sodium is likely to be a hazard to the vegetation but not the soil, although over time with low rainfall, the salinity may increase to such an extent that the plants will not thrive. The sodium ion is not attached to the soil particle (in the case of sands) and is more easily leached out of the root zone deeper in the soil profile, by rainfall or excess irrigation, where its impact on plants will be less. However, the proportion of phosphorus that is not taken up by the vegetation is also more likely to leach from the root zone and may end up in the groundwater or move sideways into creeks or watercourses. Phosphorus in waterways is a major contributor to algal growth and the potential eutrophication of the water.
On clay soils, sodium is likely to bind with the clay particles, often displacing valuable plant nutrients such as calcium and magnesium. When bound to the clay particles, sodium becomes a hazard for plants and may induce dispersion (separation of individual clay particles) in the soil. Constant wetting and drying then leads to compaction of the soil and loss of structural stability. Dispersion is a serious issue with soils and its effect is not limited to growing plants but may induce other soil stability issues that impinge upon the wider environment. Refer to the comments on the "sodium" page and note that levels of sodium greater than 20 g/wash require large land areas for disposal of greywater.
On sandy soils - sodium is not an issue with regards to structural stability. Sodium always a hazard to plants. Phosphorus leaching to waterways is a serious concern and low phosphorus detergents (preferably with no phosphorus) would be a wise choice. But then don't go an spread chemical fertiliser except at the recommended rate.
On many clay soils sodium is a real hazard for structural stability and plant growth. Always choose a low sodium detergent - or dilute and spread over a very much larger area. Phosphorus may not be a problem because of the natural ability of the soil to 'lock up' phosphorus (a complex issue beyond the scope of these pages). In these cases, phosphorus only moves with eroding particles and is not free to leach to deeper depths. Some of the detergents are sufficiently high in phosphorus as to be 'too much' for the garden in the long term. Refer to the calculation shown in the "phosphorus" page. Note the comment for levels of phosphorus greater than 1 g/wash. The higher the dose, the larger the land area required.
Interpretation
In each graph the various brands of laundry detergent powders are represented
by two bars - a blue bar representing the load of sodium in grams per
wash, and a red bar representing the phosphorus in grams per wash. The sodium loads have been ranked from lowest
(bottom of Y-axis) to highest (top of Y-axis). The phosphorus
concentrations are unranked.
All the above comments need to be considered in light of the
particular greywater reuse you wish to undertake.
You need to consider:
# the plants and their susceptibility to salts generally,
sodium in particular and maybe phosphorus - certainly high doses of phosphorus;
# the soil - sands, loams or clay - susceptible to dispersion
(a simple test is shown under the "greywater" page;
# irrigation rate on the land area available - higher rates
may require less frequent application;
# potential leaching by rainfall or excess irrigation; and
# the particular laundry detergent you prefer.
When IN DOUBT - use a low sodium, low phosphorus detergent.
ęCopyright: Lanfax Laboratories PO Box
4690 Armidale NSW 2350.
Contact: Dr Robert Patterson
+61 2 67751157 email:
lanfaxlabs@bigpond.com.au