POWDERS-NOV09
During July and August 2009, Lanfax Labs and Choice (Australian Consumers' Association) collaborated in testing 25 laundry detergent powders. Lanfax Labs provided the analysis of the laundry water when the powder was mixed at the recommended dose for both the wash only and full cycle water load of front loading and top loading washing machines. Choice undertook the performance testing of the powders according to current standards (how well the powders cleaned prepared materials). Choice has separately reported its finding in the November issue of Choice magazine (www.choice.com.au). The results of those performance tests will not be repeated here. This page will report on the analysis of the wastewater from each product and discuss their suitability for discharge as greywater.
The 25 products were sourced by Choice and samples of the detergent were received by Lanfax Labs where the recommended dose was mixed with water according to the type of washing machine. For front loaders, the wash volume was taken as 15 L and the full cycle volume as 60 L; for top loaders the wash volume was 60 L and the full cycle volume was 115 L. These volumes are typical of the current range of washing machines as set out on Water Efficiency Labelling Standards (WELS) scheme (www.waterrating .com.au) or using the data from the latest testing by Choice (www.choice.com.au).
To view the graphed data for each of the determinations in the table below, double click on the underlined title. You may need to turn to the pages "Greywater" and "Gardens" for an interpretation of the levels of each of these.
| pH | Total Dissolved Solids | Boron | Potassium |
| Sodium | Phosphorus | Sulphur | Greywater suitability |
Preparation
A sample of each detergent was mixed with deionised water (water which has
passed through mixed media that remove most of the minerals - approximates good
quality rainwater) at the equivalent rate in 1 L of water. The bottles
were shaken at 25oC for 30 minutes
Analysis
Immediately following preparation (mixing), the samples were analysed for pH, electrical
conductivity (EC), total alkalinity, turbidity, total suspended solids (TSS)
while fresh. Samples were acidified with nitric acid and analysed in a
inductively coupled plasma (ICP) for the elements boron (B), calcium (Ca),
potassium (K), magnesium (Mg), sodium (Na), phosphorus (P) and sulphur (S). By
calculation the masses of sodium, phosphorus and sulphur in a wash were
calculated.
Interpretation
Since nearly all the masses of these elements (Na, P, S) are in the wash
water, and only the residue rinsed from the clothes in the rest of the normal
program cycle (spin, spin rinse, deep rinse and spin dry) these elements are
reported in grams per wash (g/wash). Where the wash water is discharged to
sewer or septic tank, the rinse water has previously been tested and all
products had a wastewater that was suitable for greywater discharge. The same
cannot be said for the wash water only. However, collecting all the
program cycle water (wash and rinse) prior to greywater application mostly
resulted in a suitable wastewater for land application. See the results at
the end of this page for a list of those products assessed as suitable for
greywater application.
The removal of all detergent residues from the clothes depends upon the performance of the washing machine, the amount of water used to perform the rinse and the amount of detergent used in the first place. Use too much powder and the machine may not be able to remove all the residue from the clothes.
The data
In the graphs below, the products have been ranked from the lowest value (the
bottom of the Y-axis (left hand side)) to the
highest value. The length
of the bar denotes the measured value with the units along the X-axis (bottom). The longer the bar, the larger the value. The best results have the shorter bars
and they will be at the bottom of the graph. For detailed discussion of the
various parameters, you will need to see other pages on this website, or follow
the links where available.
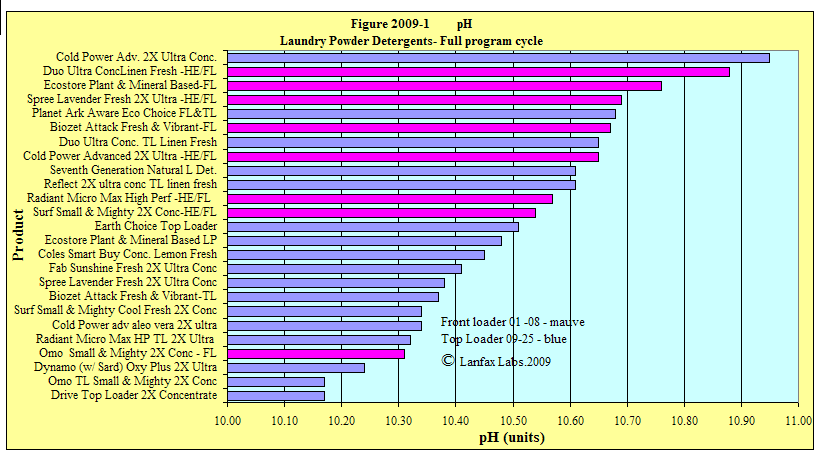
pH
A measure of the acid or alkali
nature of the laundry water. The pH of the deionised water used to mix the
detergent was 4.3, a low value because of the removal of most of the
contaminants including much of the dissolved carbon dioxide. Reticulated town
water will generally have a pH above 7. All pH values were above 10.0, therefore
classified as highly alkaline.
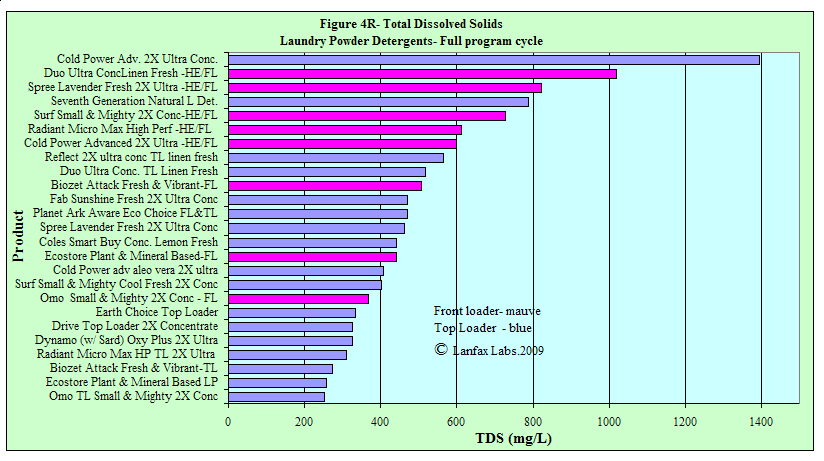
Total Dissolved Solids
This is a measure of the dissolved components in the
washing machine water. The deionised water had less than 2 mg/L, whereas
reticulated town water may have over 200 mg/L as the starting point. This value
is termed "salinity", even though it measures both "good" and "bad" dissolved
solids. If the water was evaporated, a mixture of salts would remain.
These salts then impinge upon soils and plants. The longer the bar, the greater
the potential impact of the salinity on the soil and plants.
Boron
This element, normally in the form of borax (boric acid) is no longer a major
component of laundry detergents. Of the 25 powders tested, none had more
than 1 mg B/wash. So there is no need to worry about the effects of boron on
your garden or lawns, although boron is a micro-nutrient (a nutrient required in
small amounts).
Potassium
Only three products had a potassium level over 3 g/wash. Potassium is an
ideal replacement for sodium, because potassium (K+) is an essential
plant macro-nutrient (nutrient required in large doses), whereas sodium can be
detrimental to plants and soil. If the manufacturers were to replace sodium
salts with potassium salts, the wastewater would have a beneficial value to
gardeners and community irrigation schemes.
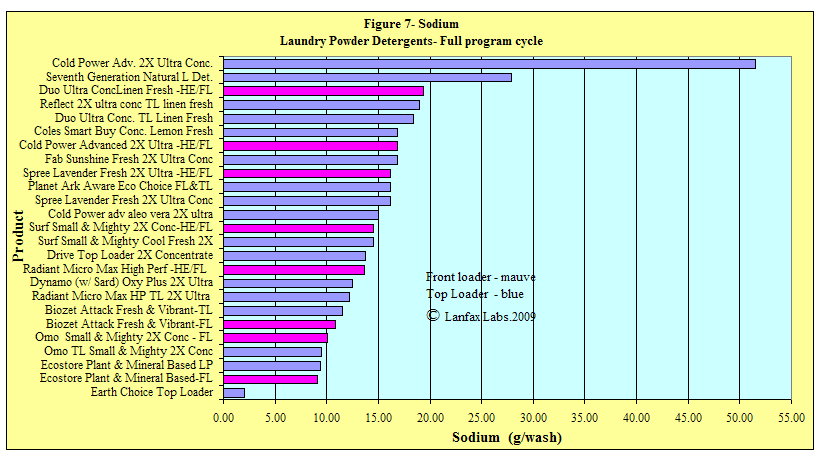
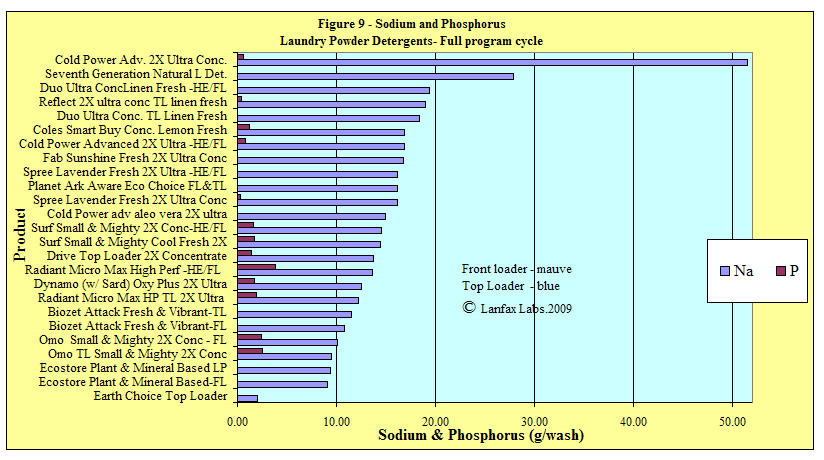
Sodium
The element sodium can be detrimental to both plants and soil contained in
irrigation water. Farmers are well aware of the environmental consequences of
sodium (referred to by the term "Sodicity") in agriculture and horticulture
while high sodicity can also play a major role in degrading engineered
structures such as buildings and roads. Since sodium is very expensive to remove
from wastewater (only by reverse osmosis), the ideal solution is to "reduce at
source" the amount of sodium in laundry detergents and other household cleaners.
As seen with the low dose products, sodium levels are generally low. Replacement
by potassium salts is a reasonable alternative.
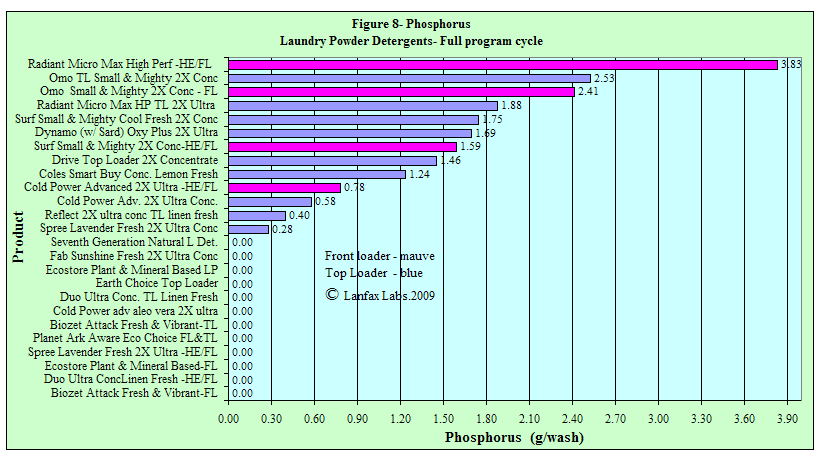
Phosphorus
While phosphorus is considered an environmentally hazardous discharge into fresh
water rivers, and lakes, P is an essential plant and animal nutrient. You cannot
survive without P in your diet and that P is only available through plants.
Unfortunately, much of the discharge of wastewater ends up in creeks, rivers and
lakes and encourages "algal blooms". For this reason the reduction in P in
laundry detergents is encouraged where the wastewater may end up in those
environments. Where the wastewater is discharged according to a sound
phosphorus balance on clay soils, the phosphorus is unlikely to reach the
groundwater, creeks or lakes. In the soil it is available as plant food.
You need to consider your receiving environment and assess whether you require a
"No P" or "low P" detergent, or whether is doesn't matter. Be aware the
phosphorus builders in laundry detergents are very efficient at deactivating
"water hardness" and that replacement compounds are likely to be less effective.
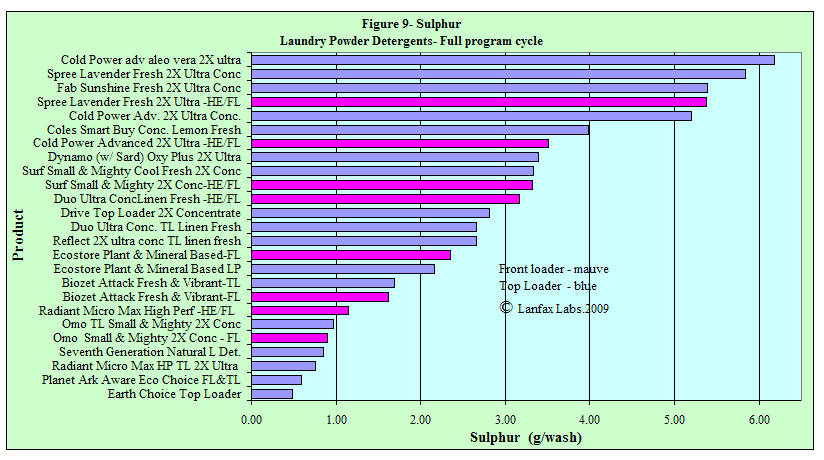
Sulphur
The reason for monitoring the sulphur levels in detergents is to gain some
understanding of the proportions of "fillers" (as sodium sulphate) that may be
used in the products. "Fillers" are used as "manufacturing agents"
(whatever that means!!) and other than making the wastewater more saline, there
doesn't really seem to be a real role for them in modern detergents, other than
making you use a cup of detergent rather than a small scoop (a mind game!!).
Sodium and phosphorus combined graph
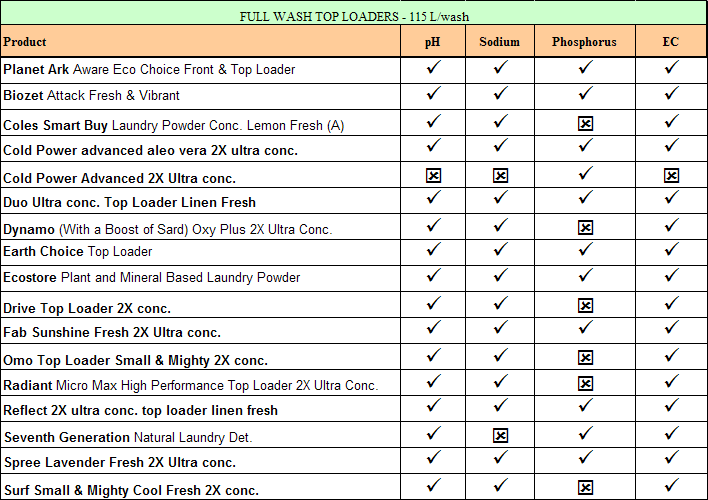
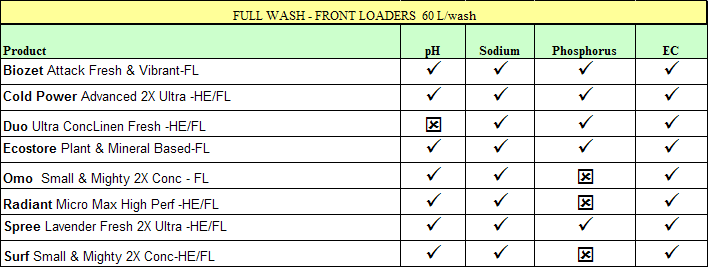
GREYWATER APPLICATION
The following table shows how each of the products was assessed against a
'greywater suitability criteria" for an irrigation area of about 150-200 m2
where the wastewater is spread evenly over the area throughout the year. Other
factors that need to be considered are the salinity of the water supply
(rainwater very low, town water highly variable), the actual dose of detergent
and whether you discharge the wash water only, the rinse water only or the water
from the whole program cycle (preferred option). See under "Greywater"
for the criteria set for greywater suitability.
| Disclaimer:
In no way does this research recommend or endorse ANY product,
rather it ranks the products for each of the analyses performed. Conclusions may
be drawn from the ranked data as explained with each graph, however, the
potential impact of the laundry products on the environment depends on many
complex inter-relationships such as: concentration at which the products are used in the wash cycle; the method of disposal of the wastewater; the receiving environment (land, river or ocean disposal); the soil loading rates and frequency of discharge; and the vegetation growing on the discharge area. |