Water Hardness
What's so important that HARDNESS needs a
page to itself?
Every wondered why the ring forms around the bath? Why the porcelain hand basin looks like it always needs cleaning? Why your hair feels dirty even when it has just been washed? There's a good reason for those outcomes and the cause is related to the chemistry of the water.
Let's start with soft water, then work our way to understanding the term 'hard water' and what it means to us.
RAIN WATER
Pure water is very difficult to maintain in that condition, simply
because gases from the atmosphere (carbon dioxide, oxygen, nitrogen oxides,
sulphur dioxide) dissolve in the water and change the chemical make-up of the
water. This difference varies with location and proximity to oceans, industry,
cities and other contributors.
Rainwater is not pure water because it has the gases and a range of other particles in the atmosphere dissolved in it, which may include sea spray from the oceans, nitrate from the electrical activity of lightning (nitrogen gas converted to nitrate), nitrogen and sulphur gases from pollution, dust, pollens, bacteria and numerous other compounds. Compared to water that has run over the ground, rainwater is reasonably clean. But you need to collect it without making it any more contaminated and therein lies a problem.
So rainwater essentially is water with some minor impurities, with an electrical conductivity (EC) less than 0.01 dS/m and a pH about 4.5 to 5.5 (that's slightly acid). Close to industrial areas, the rain may be more acid (lower pH) and can cause serious environmental problems.
Now let the rain fall on your roof. What sort of roof is it? Concrete tiles, terracotta tiles, galvanised iron, Zincalume sheeting, anodised aluminium, timber, fibrous cement, asbestos cement, lead flashings, a chimney from a wood fire, a TV aerial that is a great perch for resting birds, gutters that accumulate leaves and decay to form beaut compost? Think about it! How clean is your roof? Well, whatever is on the roof may end up in your rainwater tank and the chemicals will contaminate the water with both organic and inorganic components.
So the EC will be elevated a little, but probably still less than 0.05 dS/m and a pH a little higher around 5-6.
For the story on water hardness, we need to consider only calcium and magnesium. Hardness is an aesthetic quality of water that is used to describe the ease with which soap forms a lather. We can drink extremely hard water but it may not make a good cup of tea or coffee.
SOFT WATER
There's not going to be very much calcium or magnesium from the
dust and leaves or even from the roof tiles, and none from the steel or aluminium
sheeting. So the rainwater will be soft. There will not be enough
calcium or magnesium to form a scum with ordinary soap and certainly not enough
to need a special detergent. Sometimes from new concrete water tanks there
may be minor amounts of calcium leaching from the concrete in the first year, but certainly not
levels that would stop the water being called 'soft'.
In soft water, ordinary soap lathers quickly and easily, and the soap is easy to rinse out of clothes in the laundry or from your hair and skin under the shower. Your hair feels clean and not 'clingy'.
A problem with very soft water is that because of its purity, the copper pipes used in the house will start to dissolve in the water. The green mark on the porcelain under the dripping tap is an accumulation of copper from the pipes. The cleaner the water the more corrosive it is.
HARD WATER
We're not talking about frozen water (ice). Calcium and magnesium ions
(these are both positive ions) in water join with the soap to form as insoluble curd and reduce the effectiveness
of the soap as a washing agent. The real problem comes when you try to wash the
curd out of the clothes because as an insoluble material it sticks to the fibres of the clothes, does not re-dissolve and is left behind after rinsing.
So you may have added dirt to the clothes (in the form of these insoluble
calcium and magnesium compounds) rather than cleaning them. Clothes may feel
damp even though they are dry, and do not have a 'crispness' after washing. Over
time, this soap curd will give a dull yellow colour to white materials.
In the bath or shower, soap is difficult to get a lather with hardwater and instead of feeling slippery it seems difficult to wash. Your hair, in particular, starts to matt together and it's difficult to run your fingers through your wet hair. When your hair dries it feels sticky and clings together because the calcium and magnesium compounds have not been removed from the hair fibres but are acting like glues. Your skin may be clammy because the calcium/magnesium precipitate absorbs moisture, and may also leave the skin drier than usual.
In the bath, there are tell-tale signs of the high water mark and the hand basin gets a greasy film over the surface of the porcelain. It requires cleaning with some of the special products because this scum is insoluble in water. Shower screens and tiles get a matt appearance (dull) and appear to have a coating that will increase in thickness over time, unless removed. It is usual to use a proprietary product to remove this 'grunge'. These products are usually acidic to dissolve the calcium and magnesium precipitate.
Inside pipes hard water leaves an insoluble deposit (scale) of calcium and magnesium carbonates and over time may completely clog up the pipe. In hot water services, in the electric kettle or coffee making machines, and industrial boilers, the calcium deposits on the heating element and can build up to a stage that the water is no longer in contact with the element, the element overheats and explodes.
HOW DO WE MEASURE HARDNESS
There are two types of hardness: temporary and permanent.
Temporary hardness: This type of hardness can be removed by boiling, that promotes the formation of carbonate (insoluble) rather than the original bicarbonate (soluble) and a precipitate of calcium carbonate settle out, leaving the water 'softer' (less hard). After you let the water settle so that the calcium carbonate deposits on the bottom of the container, it is important to remove the settled carbonate - pour off the clear water, otherwise carbon dioxide from the atmosphere will dissolve in the water and cause the carbonate to redissolve.
Permanent hardness: Permanent hardness cannot be removed by boiling, usually caused by calcium and/or magnesium chlorides and/or sulphates which become more soluble as the temperature rises. This hardness can be removed through a water softener.
Total hardness:
There are some quick and simple test kits used to test for hardness
of swimming pool water. In the laboratory, the water is analysed for
calcium and magnesium and a calculation is made. Hardness can be reported for
calcium hardness, magnesium hardness or total hardness (the sum of the calcium
and magnesium hardness). Total hardness is reported in milligrams per
litre of calcium carbonate equivalent (mg/L CaCO3).
Some imported water softeners refer to hardness in obsolete German units of "degrees of hardness" (odH). To convert odH to total hardness in mg/L CaCO3, divide by 0.14. See references to other units at http://en.wikipedia.org/wiki/Hard_water)
CLASSIFICATION OF TOTAL
HARDNESS
The Australian Drinking Water Guidelines (2004) state that there are no health
guideline values for total hardness, but an aesthetic value is suggested at 200 mg/L
CaCO3 (which is reasonably hard water). Very hard water is
likely to cause scale (insoluble calcium and magnesium compounds) to form
on the inside of pipes and boilers.
Table H-1. Water hardness classification
| Classification | Calcium carbonate hardness | German degrees of hardness (dH) |
| Soft | < 60 mg/L | 0-8 |
| Good quality | 60-200 mg/L | 8-12 |
| Increased scaling problems | 200-500 mg/L | 12-18 |
| Severe scaling | >500 mg/L | 18-30 |
Other classification systems are intermediate to the Australian Drinking Water Guidelines (2004)and refer to various scales of total hardness as:
soft
<17 mg/L CaCO3
slightly hard
17-60 mg/L CaCO3
moderately hard
60-120 mg/L CaCO3
hard
120-180 mg/L CaCO3
very hard
>180 mg/L CaCO3
Obtain a copy of the Australian Drinking Water Guidelines at http://www.nhmrc.gov.au/publications/synopses/eh19syn.htm
LANGELIER SATURATION INDEX (LSI)
This index is simply a mathematical formula used
to predict the calcium carbonate stability of water. The LSI predicts that for a
particular temperature, pH, total alkalinity, electrical conductivity (as a
measure of salinity) and calcium ion concentration (mg Ca2+
/L) the likelihood of whether the water will be corrosive (negative index) or
scale forming (positive index).
Water samples analysed by Lanfax Labs will have the LSI calculated and the likely consequences reported.
SIMPLE TEST FOR HARDNESS
Take a bucket and half fill with water from the
source you wish to test. Take some ordinary soap and wash your hands in
the bucket of water, using plenty of soap. Question 1 - did the water lather
easily? Soft water lathers very easily, hard water is difficult to get to
lather. Varying degrees indicate various levels of 'softness' or 'hardness'.
Question 2 - alter allowing the water in the bucket to settle for a short time,
is there a ring of soap scum around the water's rim? In soft water the
soap will remain in suspension in the water, no scum will form. In hard
water, the formation of a 'curd' (insoluble calcium salts and the soap) around
the water's rim will be obvious.
You may also like to test the water by boiling in an open saucepan. When near to the boil, is there a froth on the surface, or a slight metallic sheen? Hard water produces either or both. When you make a cup-of-tea, is there a visible sheen on the surface of the tea (easier to see in bright light). If yes, the water is hard and will usually make a stronger cup-of-tea than soft water,
DRINKING WATER SUPPLIES
It is usual in many metropolitan and urban areas for the water
supply authorities to reduce the hardness of the water from their reservoirs so that its
aesthetic quality is improved from the raw water resource. Reducing hardness is
simply a matter of removing some of the calcium and magnesium ions, not all of
them, just enough to make the water softer. Since calcium and magnesium
rapidly form precipitates with carbonates, adding soda ash (sodium carbonate)
to water forms calcium carbonate and magnesium carbonate that then settles out as
a precipitate. Soda ash is also called 'washing soda' and its use for
normal laundry purposes is to reduce the calcium and magnesium effects on soap.
In detergents, phosphorus salts are used to isolate the calcium and magnesium in
a different way (discussed later).
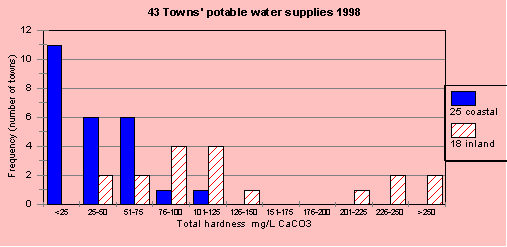
In 1998, I measured 43 town water supplies across coastal and inland NSW as part of a research project. The hardness in the water supplies was extremely variable but noticeable different between the coastal fringe and the inland (west of the Great Dividing Range). Figure H1 shows the number of towns (frequency distribution) for a range of total hardness. It is clear that inland water supplies are harder than coastal supplies. As a comparison, rainwater has a hardness of less than 5 mg/L.
Metropolitan Water Supplies
A search of the internet revealed the following levels of total
hardness in metropolitan water supplies
Table H1. Total Hardness in metropolitan water supplies (sources as shown)
| Metropolitan Supply | Total Hardness (mg/L CaCO3) | Source of Data |
| Melbourne - City West Water | 15-29 mg/L | (City West Water, 2007) |
| Melbourne | 10-26 mg/L | (Melbourne Water 2006) |
| Sydney’s water | 51-65 mg/L | (Sydney Water 2005) |
| Brisbane | 106-143 mg/L from Mt Crosby | (BCC, 2008) |
| Brisbane | 89-97 mg/L from North Pine | (BCC, 2008) |
| Adelaide | 80-157 mg/L Metropolitan Adelaide | (SA Water, 2008) |
| Adelaide | 81-140 mg/L from Hope Valley | (SA Water, 2008) |
Brisbane City Council reports on the average hardness of Brisbane's water as 68-210 mg/L CaCO3) - see the website for more details. http://www.urbanutilities.com.au/ckfinder/userfiles/files/DW%20Quality%20Data%20-%20Internet%20July2008jun2009v4final.pdf
EFFECTS OF HARDNESS ON SOAPS AND
DETERGENTS
The problem that hardness causes to our use of water for washing (persons and
clothes) is that the calcium and magnesium salts react with the surfactant (the
surface active agent) and reduce its effectiveness. We can overcome that
problem by using more washing soap to bind the calcium and magnesium. Additional
amounts of washing soda (sodium carbonate) or borax can be used to precipitate
the calcium and magnesium. The
problem with soaps in hard water is that the compounds formed with the soap
result in a greasy curd that is insoluble in water and difficult to wash out. It
doesn't matter how much extra soap we use, the curd remains insoluble.
So if we have to learn to live with hard water, how do we overcome the effects of the hardness. SIMPLE - use a high quality detergent. The harder the water, the more detergent (not soap) you use. Phosphates have been added to laundry detergents to deal with hardness and these salts are very good at combining with calcium and magnesium and keeping them in suspension so that they can be effectively washed out of the clothes. Whether substitutes for phosphorus, such as zeolites work in hard water may need to be tried. There may be an environmental consequence of removing phosphates from laundry detergents, because there has been no research on the consequences of their replacements. It is known that zeolites settle out in washing machine pipes and irrigation systems and cannot be removed by acidification, as is the practice for removing carbonate scale. Because zeolites are less effective than phosphates, we may be causing higher salinity (using more detergent) with these 'new green' detergents. The 'New Green" may not be what it is cracked up to be. Time will tell.
The third way of dealing with hard water is to pass the water through a water softener.
WATER SOFTENERS
These devices are ion exchange systems - that is, they exchange
sodium ions for calcium and magnesium ions. Water passes through a
softener, usually a large tank something like a LPG gas bottle. On its way
through the media inside the softener, the calcium and magnesium ions are
captured by the media and a replacement occurs. Sodium ions on the media
are displaced by the calcium and magnesium. Instead of the water having
say 100 mg/L (parts per million) of calcium and magnesium combined, the softened
water now has very low levels of calcium and magnesium, but levels of
sodium displaced are fractionally higher than the combined calcium and magnesium
displaced. At regular intervals, the ion exchanger has to be replenished, that
is back flushed with a brine solution (concentrated sodium chloride solution) to
replace the calcium and magnesium so the process can start again. The
backwash should be discharged to sewer and not onto the ground or into a septic
tank (or other on-site system). The period between backwash events will depend
upon the hardness of the water and the volume of water treated. Check with
your irrigation specialist for the correct sizing of a water softener.
Reverse Osmosis (RO) is the removal of contaminants from water by forcing the water through a semi-permeable membrane. These devices produce very clean water, but are expensive to operate and waste about 90% of the water passing through them. RO water is 'too pure' to drink.
Additional Information
If you have other questions that you think the answers may help other, please
email me and let me know. I cannot guarantee that I will be able to list all the
information we require to use laundry and other household products in a
responsible way. email
lanfaxlabs@bigpond.com.au
References: City West Water-Melbourne:
http://www.citywestwater.com.au/about/docs/Water_Quality_Report_2007.pdf
Sydney Water:
http://www.sydneywater.com.au/Publications/Reports/TypicalWaterAnalysis.pdf#Page=1
Brisbane Water:
http://www.brisbane.qld.gov.au/BCCWR/LIB169/june2008_CHEMICAL_ANALYSIS_WATER_QUALITY_RESULTS.PDF
(Mt Crosby and North Pine supplies, June 2008
Adelaide Water:
http://www.sawater.com.au/nr/rdonlyres/f4353c40-f966-4493-9cd1-7a14f87c8e2f/0/dwqr_0708.pdf