Salinity
Much has been written about salinity and its effects upon the environment. However, salinity is also an issue where laundry water is discharged over the soil in gardens, on lawns and in septic drainfields. A salt is simply a compound that dissociates (breaks up) in water to form positive ions (cations) and negative ions (anions). The main difference between organic compounds and salts is that while both may dissolve easily in water, the organic compound remains as an organic molecule, but the salt dissociates into cations (positively charged) and anions (negatively charged). Since most of the laundry powder is made from various salts, we need to limit the amount of salt we discharge onto land or into fresh water. Be aware, when we talk about salt we are not talking about common salt (sodium chloride) but all the salts made up from calcium, magnesium, potassium and many cations as well as the anions of sulphate, phosphate, nitrate, chloride, and carbonate.
Salinity is measured in two ways. The first is the total weight of salt used in the wash which assumes that all the components are inorganic, which is not the case for laundry detergents. This value is often difficult to derived from the information on the packet, because the usual measures are given in volumetric terms (one scoop, one cup) and not in terms of mass (weight). The greater the amount of detergent used, the higher the overall salinity. Therefore the very bulky detergents (usually the generic brands of normal powders) are likely to be the most saline by this measure.
The second method for determining salinity is to dissolve the recommended dose of detergent in the total wash water and measure the electrical conductivity (EC) using an electronic instrument.The International unit of measurement for EC is deciSiemens per metre (dS/m). The higher the salt content of the water, the greater the ability of the water to conduct electricity which is measured by the EC meter. A conversion factor is then used to allow for poorly ionised salts, to convert from EC (measured in dS/m) to grams of salt per litre. The conversion value generally accepted for water and wastewater is 680, that is multiply the EC meter reading in dS/m by 680 and call the answer milligrams per litre (mg/L).
The salinity measured in this research is for detergents dissolved in deionised water (equivalent to high quality rainwater), with a starting EC of less than 0.005 dS/m (salinity of about 3 mg/L). Many coastal town water supplies have salinity values up to about 40 mg/L while inland NSW supplies can have salinity in the clean town water up to 250 mg/L. You need to account for the salinity of the town water supply in any calculations you do to gauge possible salinity damage to your lawns, garden or subsoil drainfields.
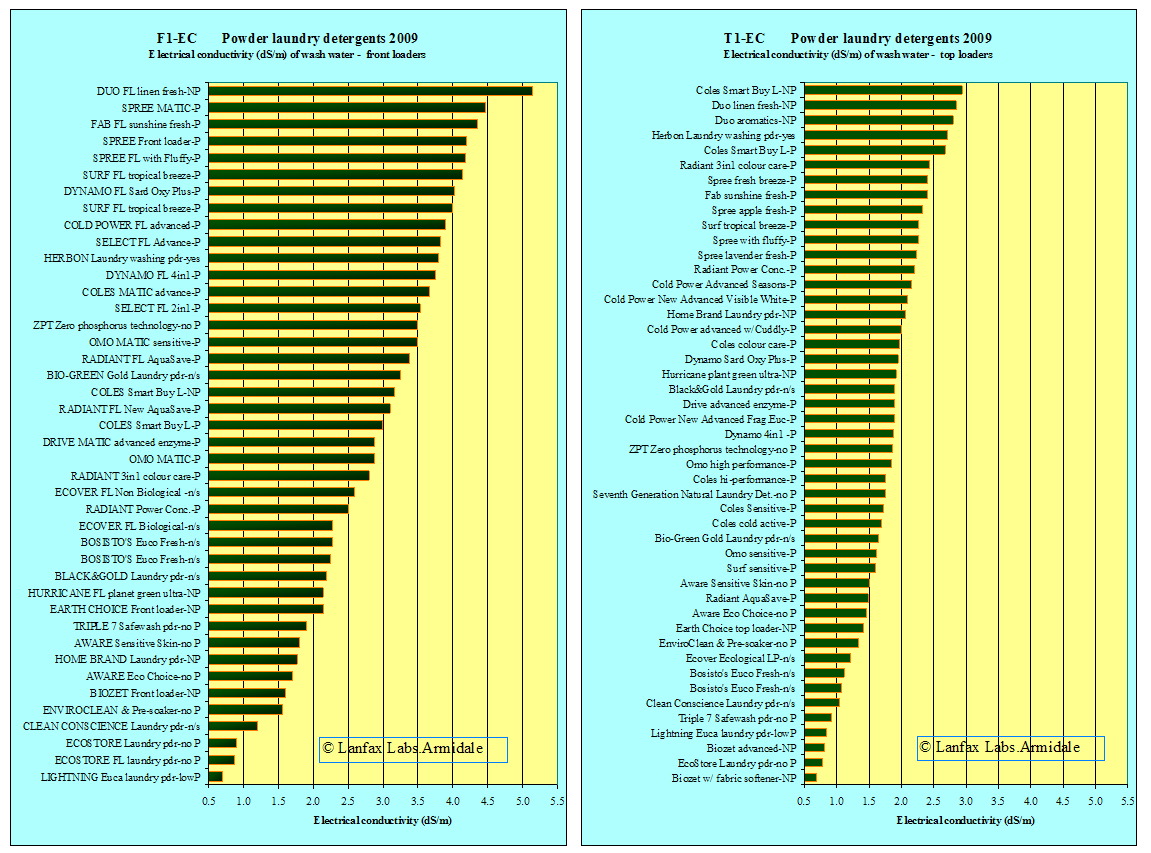
In figures F1-EC and T1-EC the X-axis scale has been kept the same so that the immediate difference in salinity levels are obvious. Of course, by diluting the wash water from front loaders (by a little more than twice) a similar EC could be met. The more the wash water is diluted, the lower the salinity.
Summary
| Source | Lowest EC (dS/m) | Average EC (dS/m) | Highest EC (dS/m) |
| EC front loader wash (42 samples) | 0.70 | 2.88 | 5.14 |
| EC front loader only (24 samples | 0.87 | 3.36 | 5.14 |
| EC top loader wash (47 samples) | 0.69 | 1.82 | 2.97 |
Assessment
The generally accepted level of
suitable irrigation water is taken as 1 dS/m while higher levels are likely to
induce some loss of plant production because of the effects of the increased
salinity on the physiology of the plant and the effect of soil salts on general
plant health. If that level was applied to the use of laundry detergents,
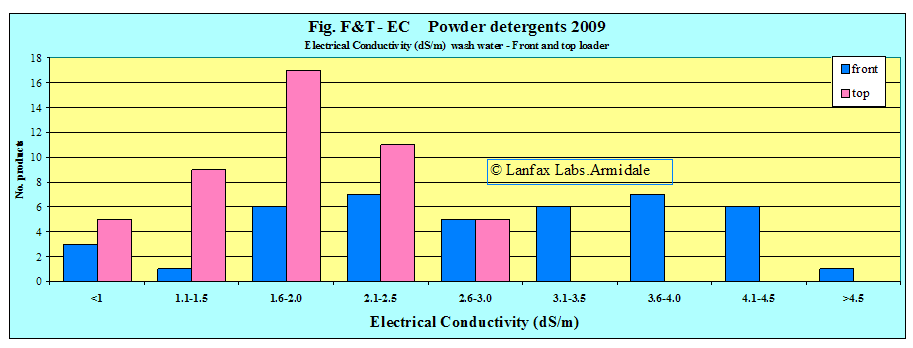
then 93% of the front loading powders exceed this level and 90% of the top
loading detergents. Figures F1-EC and T1-EC show very few detergents in the
lower ranges and most above the guidance level of 1.0 dS/m. It is clear,
however, there are some choices of low salinity products (as measured by
electrical conductivity) among the products listed above for either front
loaders or top loaders.
It is also noted that for the front loaders, many of the specific products (24) (block letters, FL or MATIC) designed for those machines fall about 2 dS/m, only two are below 2 dS/m. The highest is 5.14 dS/m and the average 3.4 dS/m. The use of the wash water as greywater from these products is NOT recommended. Similarly for both front loaders and top loaders, the popular brands are spread throughout the range.
The impact of salinity from laundry water is a concern to all end users, whether the water is discharged from municipal sewage treatment works to rivers or land application, or for those households that discharge the wastewater to on-site treatment (septic tank or aerated wastewater treatment system) and then to land application (above or below ground). Unfortunately, because the large metropolitan areas discharge to ocean outfall and "waste" all the valuable components in the water, the effects of salinity from laundry detergents appears not to concern government regulators. Your actions need to consider the consequences of salinity on your land application area, choose products that have low salinity - and there are plenty of choices! You may have to use 2 dS/m as the threshold and spread the greywater over a larger area, or dilute the wash water with rinse water prior to irrigation.
Remember: Electrical conductivity measures all the ionised components from the salts in the detergents. While some elements may be important plant nutrients, in laundry detergents most of the positive ions are from sodium. Therein lies the real problem. Now read on to the section on sodium to see that measurement of total salinity may not be a good indicator of potential or plant problems
ęCopyright: Lanfax Laboratories PO Box
4690 Armidale NSW 2350.
Contact: Dr Robert Patterson
+61 2 67751157 email:
lanfaxlabs@bigpond.com.au