Sodium
The effects of salinity cover the general ionised components of the laundry
water (cations and anions of all the salts) that together can affect the
normal functioning of plants (by upsetting their water balance), change the
availability of other plant nutrients (nutrient imbalance) or lead to a hostile
soil environment with changed structural stability. However, some salts
are beneficial and are used as a remedy to overcome soil problems. Heavy
clay soils can be ameliorated with lime or dolomite (also increases pH) or
gypsum (no change to pH), but these products significantly change the soil
salinity status. Fortunately, the majority of the cations are calcium or
magnesium and these elements are essential plant nutrients.
Unfortunately for laundry product manufacturers, sodium salts have the best and the worst characteristics. All sodium salts are highly soluble, hence of a benefit when manufacturers want their products to rapidly dissolve in cold water. Sodium (as the cation) is used in various combinations with anions to form highly soluble salts. Phosphorus is often carried as sodium tripolyphosphate, the sodium part making it highly soluble. Washing soda is sodium carbonate, a highly soluble, yet highly alkaline product.
Secondly, because sodium can be detrimental to plant physiology (affects osmotic pressure in plant cells) and lead to loss of soil structure, the amount of sodium in laundry detergents needs to be limited when the wastewater is to be discharge to vegetation or soil absorption areas. When wastewater is discharged as "ocean outfall' (the dark ages approach to sewage disposal) the sodium concentration is irrelevant, but the loss of phosphorus to the oceans is an obscene waste of a limited, non-renewable resource (environmental vandalism).
When a sodium salt is dissolved in water it ionises to sodium ions (Na+) and the other part as an anion (negative charge) so that the sodium then acts independently of the anion, although influenced by salinity and pH. Take for example sodium tripolyphosphate which is extremely soluble. When dissolved in water it ionises to sodium cations and phosphate anions in equal numbers. The sodium cations act independently of the phosphate anions.
Sodium salts can never be biodegradable because they are inorganic compounds. So the label about biodegradability does not apply to any of the sodium salts in the laundry detergent.
Since the only guide to the 'desirable' upper limit of sodium is based upon loss of plant production, an other term "sodium adsorption ratio" is in common use. This term, when stated simply is a measure of the proportion of sodium relative to beneficial elements calcium and magnesium (the ratio is complex and beyond the scope of this section). See also discussion under the heading "sodium adsorption ratio".
Interpretation
To interpret the graphs, the shorter the bar the better for soils that may be
adversely affected by sodium (clay loams and clays), or plants that are
sensitive to sodium. See advice from your horticulturalist is in doubt. As a
means of evaluating the potential risk to your soil, you need to estimate the
area of garden or lawn over which you intend to spread the wastewater, then
calculate the annual load of sodium as a salt load per hectare equivalent. See
the calculation below.
Because sodium is very difficult to remove from wastewater, prevention is better than cure. Consider using products that are low in sodium. Note that concentrated powders have a lower sodium level because of the lower use of "fillers".
It is interesting to note that the products specially formulated for front loaders have high levels of sodium as do many of the generic brands. It can be interpreted from this that these products are more environmentally hazardous than those with very short bars.
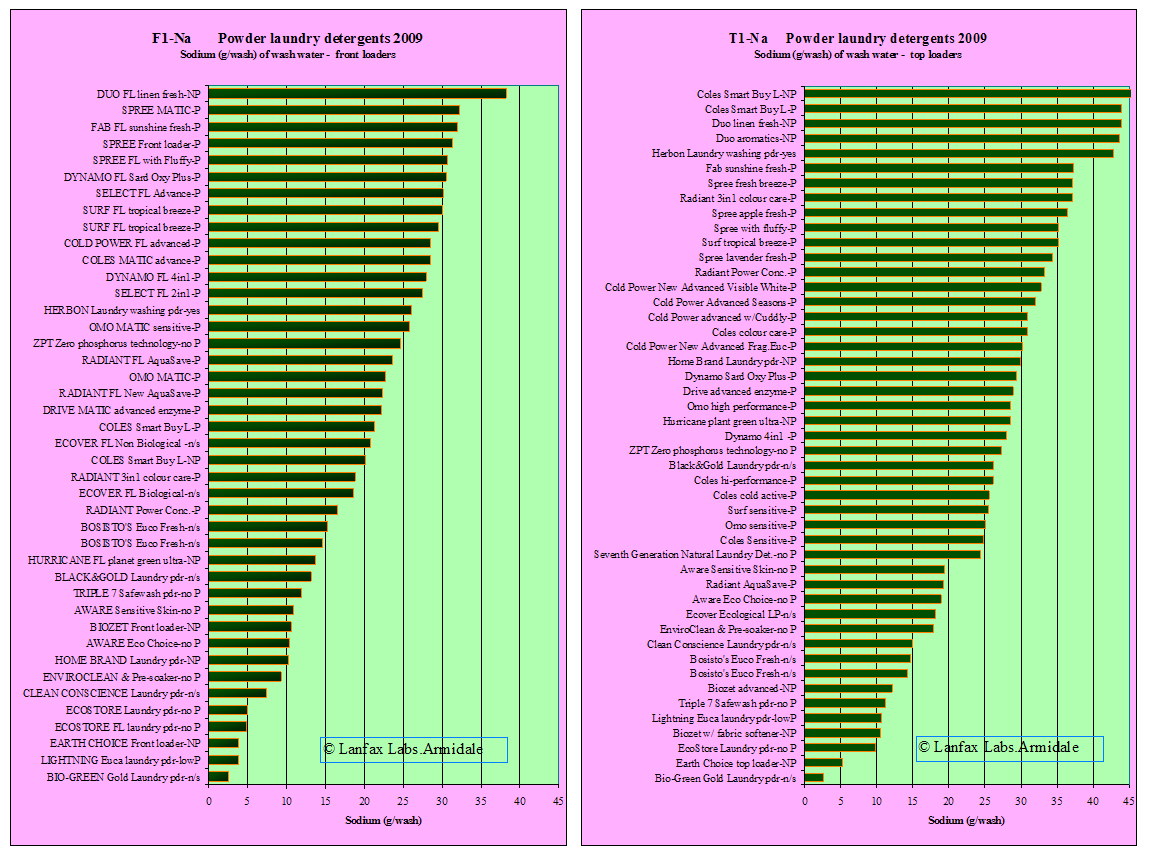
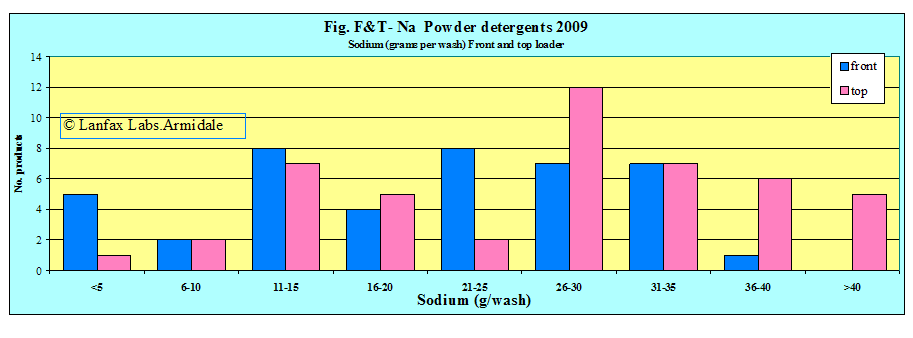
Summary
| Source | Lowest Na value (g/wash) | Average Na value(g/wash) | Highest Na value(g/wash) |
| Sodium front loader wash (42 samples) | 2.5 | 19.7 | 38.3 |
| sodium front loader specific (24 samples) | 3.8 | 24.4 | 38.3 |
| sodium top loader wash (47 samples) | 2.6 | 26.4 | 45.4 |
Assessment
The assessment of laundry detergents based upon the sodium concentration is
one which has not been addressed by the product manufacturers themselves, or the
industry regulators, or the municipal sewage treatment plant operators. It
is clear that whatever the manufacturers want to make, however they market their
products and how they are used in homes across the world is something that we
should just accept as driven by market forces. It is here that I am in absolute
disagreement. I consider many of the products listed above should be
removed from sale as they have the potential to seriously degrade our land
environment. Sodium in large concentrations is detrimental to plants and
soil. Simple demonstrations can show the effect of laundry detergents on a
range of soil - results visible in a couple of hours, do not have to wait
years for the actions to be clearly seen.
If the 'acceptable upper limit' is 20 grams per wash (this must include sodium from the source water), then 55% of the front loading detergents are above this limit and 68% of top loading detergents exceed this value. The greater volume of water from the top loaders give the ability to spread the load of sodium (g/wash) over a greater area. One is limited by the smaller volume of water in front loaders and distributing the sodium load over a large area becomes difficult (mostly likely not done).
The solution is simple. Test out a small section of your garden or lawn to see what happens when you discharge laundry water. Check on the condition of the plants (do they look healthy?) and see what happens to the soil. You can check to see what the soil looks like when you dig up a small section - if it looks to be clumping together - you may have a problem. If the surface looks like its covered with a fine sheet of clays, you again have problems.
Calculation of Sodium per wash
Sodium concentration in the wash water is translated to sodium in the
irrigation area by multiplying the concentration (mg/L) by the volume of water
(L) from the
wash cycle. Front loaders use less water in the wash cycle than top
loaders for the same size of wash - this is may vary considerably across
the various brands and how the washers are used in daily activities.
| Laundry product's sodium load (from graph F1-Na, T1-Na) | 20 grams per wash |
| Number of washes per week | 7 |
| Number of washes per year | 365 |
| Sodium load each year (wash load X number of washes per year) | 20 g x 365 = 7300 g = 7.3 kg (1000 g = 1 kg) |
| Area to be irrigation with washing machine water over the whole year | 100 m2 |
| Actual load in kg/ha (sodium load divide by area and multiplied by 10000) | 730 kg/ha |
| Convert sodium load to salt load (divide by 0.4) | 730 / 0.4 = 1825 kg salt per hectare. (See explanation below) |
| Assessment (if salt load greater than 1000, need to increase area) | require at least 180 m2 to safely distribute that load |
Note: A salt load of 1 tonne per hectare is considered in agricultural enterprises to be level at which the productivity of plants decreases, that is, there is a loss of vegetation because of the stresses introduced into the environment around the plant. While this is a simplistic approach, it is one which provides a rough assessment of the quality of irrigation water. The conversion of 0.4 from sodium to salt is to account for the proportion of sodium in sodium chloride.
ęCopyright: Lanfax Laboratories PO Box
4690 Armidale NSW 2350.
Contact: Dr Robert Patterson
+61 2 67751157 email:
lanfaxlabs@bigpond.com.au